Challenges And Opportunities In Medical Device Manufacturing
Shalane Layugan July 15, 2021
Encompassing items as simple as bandages all the way through to ultra-high-tech pacemakers, the U.S. medical device market is one of the largest in the world. Forbes called it a “disruptive” market of $410 billion through 2023. Today, “disruptive” is an appropriate word.
More than a decade ago, the medical device manufacturing industry saw an increasing emphasis on design and simplicity, driven by software and technologies. And innovation contributed to a shift in power from the pharmaceutical to the medical device industry. These days, there is still a high demand for technological, intuitive devices — with a faster time-to-market than ever before. Hospitals, medical facilities, and other markets are pressuring medical device manufacturers to be more secure, innovative, cost-effective, and efficient. What are some challenges and opportunities for manufacturers in the medical device industry?
Technology And Security
The fourth industrial revolution, known mainly as Industry 4.0, introduced manufacturing to a world of state-of-the-art digitalization, like IoT, robotics, and artificial intelligence. It also raised a pressing concern for cybersecurity.
Cybersecurity of medical devices became more of a concern after an attack on the National Healthcare Service in 2017 and paralyzed U.K. healthcare computer systems. In 2018, the FDA recalled two healthcare defibrillator models after finding a potential security breach in systems. The vulnerabilities could allow a hacker to access the device and possibly deplete the battery or issue impromptu cardiac commands.
Threats and vulnerabilities in any business situation can never be completely eliminated. However, with an increase in technological security threats affecting product quality, contributing to high recall rates, and introducing the likelihood of patient harm, the FDA has placed mandatory reporting tools in place to monitor device performance and detect potential issues. There are pre and post-market cybersecurity guidances manufacturers must address — meaning, it is the responsibility of the manufacturer to test the product before releasing it and implement patches after product release too. Manufacturers simply must demonstrate their products are safe and reliable throughout their lifecycle, so continuous and efficient monitoring is imperative.
Learn More: Information Technology Challenges In Manufacturing
Regulations And Government
Under the Medical Device Reporting (MDR) regulation, manufacturers are required to report certain adverse events or product problems to the FDA about medical devices. The MDR has also evolved in Europe to ensure a consistently high level of public health and patient safety standard — for example, manufacturers are required to collect data about their performance to improve transparency.
Notably, critiques of the medical device regulations from the FDA and EU are conflicting — that regulatory requirements are so complex they hinder device availability to the public and yet regulatory requirements that are not strong enough put citizens at risk. The healthcare environment is complex and the supply chain logistics in the medical and pharmaceutical industries are critical to any emergency health situation.
Manufacturers should act on the opportunity to streamline operations and improve data management to not only build stronger, more collaborative relationships throughout the supply chain but to create efficient responses in times of need. Pharmaceuticals, medical device manufacturers, hospitals, and health facilities must work together to navigate regulations and manage risks throughout every step of the quality control process and product lifestyle.
For example, health care delivery organizations and manufacturers must collaborate closely to communicate any necessary changes to devices like new patches or network upgrades — it is not a particular responsibility of one party over the other, but a joint responsibility.
Related: The Internet of Things Challenges For The Supply Chain
Product Design Processes And Development
While continuous innovation supports new product design and development, the added impact of regulations on the manufacturing sector results in simpler administrative tasks and increased legal certainty and credibility of the overall medical device system.
Manufacturers should see this as an opportunity to improve their processes while staying ahead of competitors. A top medical device company reported an annual cost reduction of $1.2 million within two years after implementing an electronic trial master file (eTMF) system — a process where documentation is moved from paper to electronic.
Medical device regulations and technological advancements are constantly evolving. Manufacturers should implement a data management strategy that provides a complete view of data to help with ongoing compliance and regulations. Data-driven decision-making would be more impactful to inspections and the supply chain — and many processes could even be automated with digital transformation solutions.
Dive Deeper: Identifying Weaknesses, Using Data To Strengthen The Supply Chain [Q&A]
Marketing Strategy For New Medical Device Product Launch
If you're developing a new medical component or device, it's just as important to prepare a framework for your product launch. Research before you do anything else – according to studies, as many as 95% of new products fail to make significant sales. Ensure your components or special parts go through testing, performance trials, and are properly certified for the medical industry.
Jump Start Your Product Development With These B2B Resources:
- The 2021 Industrial Buyer's Search Habits Survey Results
- What Are The Top Sourced Materials & Products? - 2021 Sourcing Activity Snapshot
- The State Of North American Manufacturing 2021 Report
Showcase your certifications, capabilities, and products on your website so buyers in the medical industry can evaluate you more easily— more than 70% of B2B buyers are researching suppliers online. Announce any relevant product updates in email campaigns so buyers keep your business top of mind and return as repeat customers. (See 9 Email Types You Should Be Sending Here.) B2B buyers and procurement managers are interested in how your solutions solve their problems, machining tolerances, quality control, and any engineering advice you have to offer. They want to see you have a proven track record in their industry.
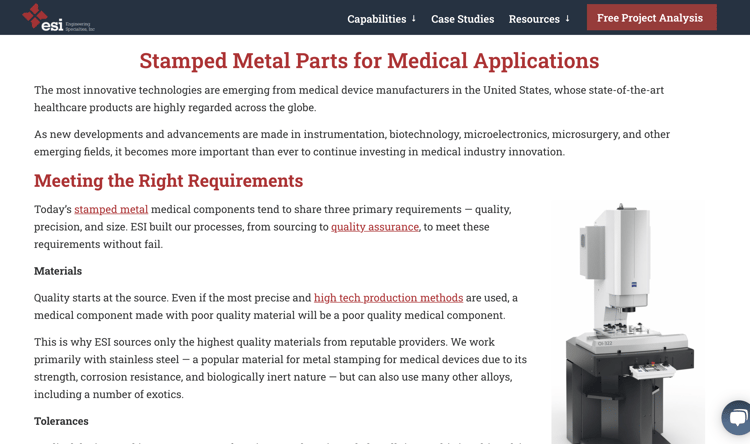
Engineering Specialties, Inc. (ESI) is a custom manufacturer of metal stamped parts, components, and assemblies. Their website features an "Industries Served" section highlighting their investment in manufacturing innovation across many markets. In the example below, their Medical Industry page details how they can meet the right requirements, quality materials, tolerances, and more.
"We tried other people with our website and marketing but found their focus wasn't on manufacturing, and Thomas is. We get a lot of activity now and steady results weekly — a lot of inquiries thanks to Thomas and our online marketing efforts," Ron Delfini, President at Engineering Specialties, Inc.
Curious to see how your website and online efforts are faring against your competitors? Request a free digital health check and we'll assess your website and let you know exactly what you need to target the industries you want to do business with.

Solve Your Manufacturing Challenges Today
Deloitte’s 2018 survey of U.S. healthcare consumers and physicians discovered more than half of consumers use technology to track their health. Meanwhile, only 9 percent of providers have implemented technology for remote monitoring or have integration of data from wearables, and just 27 percent of providers intended to add this capability by 2020. Today’s regulations and data being collected not only enable the manufacturing sector to produce safer, more effective innovative devices, but should be a signal for manufacturers to provide robust and reliable evidence that their devices can reduce costs, improve efficiencies, and lead to better patient outcomes.
Public and private collaboration can be extremely effective in solving health and safety challenges, and it’s more important now than ever in today’s digital world to maintain trust and transparency. Thomas has been powering the manufacturing and industrial space for more than 122 years, so we understand firsthand what it takes to innovate through challenging times. Contact us to get started on targeting more customers in the industries you want to do business with.
Manufacturing company profiles on the Thomas Network let the medical industry (and other industries) partner with the right suppliers fast.
For more manufacturing insight to solve your challenges, visit the resources below:
- See what companies from other industries are searching for your products and services with our free prospect report.
- 2021 Q1 Sourcing Activity Data: It’s no surprise that medical equipment and supplies are on the list of Top 20 Products sourced on Thomasnet.com last quarter. Find out the other top products are services in our quarterly activity snapshot.
- Blog: Continuing To Promote Your Manufacturing Business During An Economic Downturn
- Join other pharmaceutical and medical companies utilizing digital advertising to grow their business with a free company profile on Thomasnet.com to get seen by more buyers looking to source North American suppliers.
- Request a free Digital Health Check and receive an analysis of how your online presence compares with competitors and find out exactly what you can improve to get more buyers.
- Subscribe to our email newsletter, Thomas Industry Update, to get daily news like these straight to your inbox specifically for manufacturing and industrial companies.
Other Industry-Specific Blogs:
- Challenges And Opportunities In The Aerospace Industry
- Opportunities For Manufacturers In Solar Energy Equipment
- Challenges And Opportunities In The Pharmaceutical Industry
- Opportunities For Lithium-Ion Battery Manufacturers
- Challenges And Opportunities In Private Labeling
- Challenges And Opportunities In Cannabis Industry
- How To Grow Your Steel And Metals Company
- Challenges And Opportunities In The Food And Beverage Industry
- How To Grow Your Textiles Manufacturing Company
- 11 Tips For Growing Your CNC Machining Business
- How Can Private Label Manufacturers Increase Sales
Did you find this useful?











